Abstract
Nutritional supplements may be important on cognition but the evidence is heterogeneous. This meta-analysis aimed (1) to determine whether nutritional supplements provided to pregnant women or young children could improve cognitive development of children in developing countries, and (2) to explore how supplementation characteristics could improve children’s cognitive outcomes. This meta-analysis examined nutritional supplementation studies in 9 electronic databases and 13 specialist websites. Experimental studies were included if they were published from 1992 to 2016, were conducted in developing countries, had nutritional supplementation for pregnant women or children aged ≤8, and reported effect sizes on cognitive outcomes. Interventions with confounded components, such as stimulation and parenting, were excluded. 67 interventions (48 studies) for 29814 children from 20 developing countries were evaluated. Childhood nutritional supplementation could improve children’s cognitive development (d 0.08, 95% CI 0.03–0.13) and those with ≥5 nutrients was particularly beneficial (0.15, 0.08–0.22). Antenatal supplementation did not improve cognitive development (0.02, -0.01 to 0.06) except for those implemented in the first trimester (0.15, 0.03–0.28). In conclusion, childhood nutritional supplementation was beneficial to cognitive development but could be optimised by providing multiple nutrients; antenatal supplementation should target pregnancy women in the first trimester for better cognitive benefits.
Similar content being viewed by others
Introduction
Pregnant women and children under five are particularly vulnerable to micronutrient deficiencies (MND)1. As a response, the World Health Organization (WHO) has issued a series of guidelines on nutritional supplementation for pregnant women and young children2. Despite great efforts in the past decades, childhood undernutrition is still prevalent in developing countries3.
Nutritional supplements not only can improve growth and physical health of children in developing countries4, and are also important to their early development, including cognition5,6,7. The achievement of optimal early development is crucial, because it could reliably predict later health, education, and well-being8, 9.
Previous research suggests childhood supplementation that included iron and long-chain polyunsaturated fatty acids had minimal effects on cognitive performance10, 11, while supplementation with multiple micronutrients or adding multiple micronutrients to food appeared to have a benefit12. A recent meta-analysis has found a small but significant effect of nutritional supplements on child development13 and another systematic review reported that the combination of stimulation and nutritional supplementation benefited development14. However, the first meta-analysis did not analyse how intervention characteristics (e.g. types and quantities of supplements) may affect the cognitive benefits, and the second review did not provide any quantitative synthesis of data. Another meta-analysis investigated the benefits of nutrition interventions on cognitive development of children under two; this identified a small effect for postnatal intervention but a null effect for antenatal intervention15. Focusing on children under two allowed a more homogenous analysis, but the effect on higher-order cognitive performance, such as literacy and numeracy, cannot be studied. Furthermore, a Cochrane review has found that food supplementation’s effect on childhood cognitive development was mixed, while that for psychomotor development was medium sized, even though only two studies were synthesised16. This review has conducted a systematic subgroup analysis, which are useful for future intervention planning. Nevertheless, it only focused on food supplementation, limiting its applicability to nutritional supplementation in general.
The current meta-analysis combined a new systematic literature search (for articles in 2013–2016) with the review commissioned by Department for International Development, UK (DFID, for articles in 1992–2012) with the following aims: (1) to examine to what extent did pure nutritional supplementation (i.e. the only difference between the intervention and control arms was nutritional supplementation, but not stimulation, parenting, or cash transfer, etc.) improve cognitive development of young children in developing countries, and (2) to examine the influence of supplementation characteristics (i.e. timing of intervention, types and quantity of nutrients, and duration of follow-up) on intervention benefits.
Results
Study identification
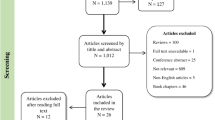
The original DFID study identified 25 studies published from 1992 to 2012. Details of the literature search of the DFID study can be found in the Appendix Fig. 1. The new literature search identified 548 studies published from 2013 to 2016. After iterative screening on study title, abstract, and full text, 525 studies were excluded, most commonly because they were observational/review studies, conducted outside developing countries, did not report/measure cognitive outcomes, or targeted children aged >8 years (Fig. 1). The new literature search, therefore, included 23 studies eligible for this meta-analysis. Adding this to the original DFID review, there were a total of 48 studies included, covering the period from 1992 to 2016.
The 48 included studies were conducted in 20 developing countries: Bangladesh, Chile, China, Colombia, Gambia, Ghana, Guatemala, India, Indonesia, Jamaica, Malawi, Mexico, Nepal, Pakistan, Peru, South Africa, Tanzania, Thailand, Viet Nam, and Zambia. All studies except threeS16, S27, S45 were randomised controlled trials. Five studies targeted at-risk groups, including full-term low-birth-weight,S37 low weight-for-age,S41 low height-for-age,S17, S18, S24 and iron deficiency anaemia.S10, S15 These conditions were included in this meta-analysis because of their prevalence in developing countries.
We identified 67 interventions from the 48 studies: 44 on childhood supplementation, 19 on antenatal nutritional supplements, and 4 on both antenatal and childhood supplements. A total of 16944 and 12870 children were in the intervention and control arms respectively (See Appendix Table 1 for detailed information on the studies and interventions).
Cognitive benefits of nutritional supplementation
There were 48 childhood and 23 antenatal nutritional interventions (including the three with both antenatal and childhood supplements). We found a significant pooled effect size of 0.08 (n = 48, 95% CI 0.03–0.13, p = 0.002; Fig. 2) with moderate heterogeneity (I2 53.97, p < 0.0001) in childhood interventions, and a pooled effect size of 0.02 (n = 23, 95% CI −0.01 to 0.06, p = 0.20; Fig. 3) with moderate heterogeneity (I2 47.45, p < 0.0001) in antenatal interventions. Excluding the studies with no random treatment allocations, the pooled effect size for childhood supplementation was slightly lower but was still significant (n = 44, d 0.05, 95% CI 0.004–0.10, p = 0.03).
Childhood nutritional supplements included iron (25 interventions), zinc (23), folic acid (18), lipid/fat (13), calcium (14), vitamin A (14), vitamin B2 (13), protein (11), and vitamins B1, B3, B12 (9). Antenatal nutritional supplements given to mothers during pregnancy included zinc (15), iron (10), vitamin A (10), vitamins B1, B2, B3, B12 (10), vitamin C (9), and iodine and selenium (8).
Moderation analysis
The number of nutrients provided was significantly associated with the supplementation benefits (Table 1). Childhood supplementation which included five or more different nutrients produced a significantly larger effect size of 0.15 (n = 16, 95% CI 0.08–0.22, p < 0.0001) compared with 0.02 (n = 15, 95% CI −0.05 to 0.09, p = 0.50) from single-nutrient supplementation (p = 0.005). A similar pattern was observed in antenatal supplementation with marginal statistical significance (p = 0.0496).
Timing of nutritional supplements was also associated with the cognitive benefits of supplementation (Table 1). Antenatal supplementation appeared to have the strongest benefit when started in the first trimester (n = 4, d 0.15, 95% CI 0.03–0.28, p = 0.02); supplementation started later did not yield any significant benefits. Similarly, supplementation on children aged 6–18 months had significant benefit (n = 27, d 0.09, 95%CI 0.02–0.15, p = 0.009) whereas those of older children did not (n = 15, d 0.04, 95% CI −0.06 to 0.16, p = 0.37).
Duration of follow-up (i.e. the time between intervention completion and outcome assessment) was significantly associated with the benefits of antenatal supplementation. Interventions with ≥5 years of follow-up had significantly stronger benefits (n = 5, d 0.10, 95% CI 0.02–0.19, p = 0.02) than the others (n = 19, d 0.005, 95% CI −0.04 to 0.05, p = 0.94). Such phenomenon was not observed in childhood supplementation.
In childhood interventions, several nutrient types were associated with cognitive benefits: iron (n = 25, d 0.09, 95% CI 0.03–0.15, p = 0.01; Table 2), zinc (n = 23, d 0.09, 95% CI 0.02–0.15, p = 0.01), calcium (n = 14, d 0.14, 95% CI 0.07–0.21, p = 0.0002), vitamin B2 (n = 13, d 0.11, 95% CI 0.03–0.19, p = 0.01), and protein (n = 11, d 0.13, 95% CI 0.03–0.22, p = 0.01). On the other hand, antenatal supplementation was found effective when they had iron (n = 10, d 0.05, 95% CI 0.002–0.10, p = 0.04), vitamins B1, B2, B3, B12 (n = 10, d 0.05, 95% CI 0.01–0.10, p = 0.02), and vitamin C (n = 9, d 0.07, 95% CI 0.02–0.12, p = 0.004).
Funnel plots and Egger regression tests showed no publication bias in both childhood and antenatal supplementation (z 0.64 and −0.006, p = 0.52 and 1.00; Appendix Figs 2 and 3).
Discussion
In our meta-analysis of 67 interventions, childhood nutritional supplementation was found to be generally effective in improving cognitive development of children in developing countries. This finding was also supported by the previous preliminary evidence13. Even more importantly, we found that timing of supplementation, number of nutrients, and some specific types of nutrients were associated with better effectiveness.
Number of nutrients seemed to be a crucial factor for optimal cognitive development. Supplementation with five or more nutrients had much stronger benefits than those with single nutrient. This could be related to the fact that multiple nutritional deficiency or insufficiency is relatively common in developing countries17. Provision of multiple nutrients is more likely to bridge the gap and prepare the optimal foundation for rapid brain development in early childhood.
Childhood supplementation of iron, zinc, calcium, vitamins B2 and proteins were found to be particularly effective in improving cognitive outcomes. Although the exact mechanisms are still poorly understood, this could be related to the roles of these nutrients in early brain development18. For example, protein plays a critical role in brain growth and advancement in cognitive abilities6, and vitamin B2 (riboflavin) is required for metabolising fatty acids by brain issues19, 20, which are essential for brain development.
Timing of nutritional implementation appeared to be important to cognitive outcomes as well. Supplementation programmes implemented from 6 to 18 months of age had the largest benefit among all age groups. The supplementation of nutrients to young, malnourished children may provide them with necessary resources for rapid brain development during the first year of age. This important finding on timing of intervention should be factored into future programme design21.
The supplementation for children aged 18 months and above were found to be ineffective to their cognitive outcomes. Nonetheless, we should be cautious that this group of children were assessed with heterogenous measurements. Some were assessed with cognitive/executive function tests, while some with school readiness/achievement. We should also note that many of these studies only provided single or relatively few nutrients.S41, S44, S47 Supplementation provided multi-nutrient food fortificationS42, S43 or early primary school feedingS45 did achieve a small to medium effects (0.17–0.30) for this group of children, even though a study supplementing multi-nutrient powderS46 did not have significant effect (−0.07). This finding is consistent with the current understanding of brain development in the early ages. While our brain is developed most rapidly in the first year of life, the neural connections for higher cognitive circuits are still actively formed throughout the earlier years until middle childhood22. To understand what kind of supplementation would benefit these older children, future trials may consider multi-nutrient food fortification and feeding programmes.
Consistent with previous studies on antenatal supplementation23, we found minimal effects of antenatal nutrition supplementation on children’s cognitive development. However, pre-specified subgroup analysis showed that nutritional supplementation in first trimester of pregnancy actually benefited children’s cognitive development. This finding suggests that some of the previous trials may have missed the critical window for intervention in early pregnancy.
It is also worth noting that this meta-analysis may have underestimated the true potential of antenatal supplementation, as most studies included had only short-term assessments but the true benefits of supplementation may only become apparent after a longer period. In this meta-analysis, the long-term cognitive functions assessed five years after antenatal supplementation showed a much larger effect size, more than twice of those with short-term assessment. This evidence echoes with the conclusion of Copenhagen Consensus that nutritional interventions had substantial benefits to children’s cognitive development and long-term productivity, which could yield a high rate of economic return24.
The following limitations should be noted. First, three of the studies in the meta-analysis were not randomised controlled trials. There may have potential bias related to underlying confounders. However, it should not substantially affect our results and conclusion given their small proportion in the whole meta-analysis. Second, the exact timing of cognitive assessments varied among studies which may make comparison of outcomes difficult. However, most studies assessed children’s cognitive performance soon after the programme and within one year of the supplementation. Meta-regression analysis was also conducted to address the potential heterogeneity due to the timing of assessment. Third, details on the participants’ baseline cognitive abilities and compliance to nutritional supplementation were not available in most publications. Future trials should pay more attention in reporting this important information. Fourth, the analysis of dosage cannot be reliably conducted because some studies did not report the dosage used and some had time-varying dosage. Fifth, some of the moderation analysis were possibly underpowered (e.g. quantity of nutrients for antenatal supplementation), so the statistical insignificance did not necessarily prove a null effect. Sixth, keywords such as ‘developing country’ and ‘low- and middle-income country’ were used to limit the number of search results, which could have excluded some relevant studies. Findings of some specific nutrients, such as iodine, may not be complete. Last but not least, the study focused on early interventions and excluded most of the school feeding programmes. The impact of these school feeding programmes need to be studied in a future meta-analysis using a broader age criterion.
In conclusion, this meta-analysis provides robust evidence on the benefits of nutritional supplements on children’s cognitive outcomes in developing countries. Future supplementation should aim to provide multiple nutrients to younger children and pregnancy women at the first trimester. To identify the optimal supplementation, future studies should include both short- and long-term assessments on the cognitive performance of children and consider the potential interactive effects of different nutrients.
Methods
The current study was an extension of a literature review commissioned by the Department for International Development, UK (DFID) in 2013, which included searching of studies published from 1 January 1992 to 31 December 2012. Detailed methodology of that commissioned study was published elsewhere25, which focused on the cognitive benefits of early childhood development programmes. The present study further reviewed the original studies and extracted additional information that were not available or reported previously4. This study also searched for nutritional supplementation interventions published from 1 January 2013 to 31 December 2016, conducted original analysis, and reported new findings, including the separation of antenatal from childhood nutritional supplementation and the investigation of supplementation programme characteristics in detail.
Study identification strategy and selection criteria
The systematic review and meta-analysis was conducted in accordance with PRISMA guidelines. The systematic literature search examined quantitative evidence on the benefits of nutritional supplementation implemented from pregnancy to 8 years of age on cognitive development in children in developing countries.
The identification of studies followed the Evidence for Policy and Practice Information and Co-ordinating Centre’s guidelines26 using four approaches: searching electronic databases, manually searching key journals, searching specialist websites, and asking experts in the field. Nine electronic databases were searched by specific keywords in the original DFID study: Academic Search Elite/EBSCOhost, Cochrane Reviews, Google Scholar, JSTOR, ProQuest, PubMed, PsycINFO, The University of Hong Kong Libraries Catalogue, and Web of Science. Keywords reflecting early childhood interventions included early childhood programme, early intervention, and early nutritional supplementation. Keywords reflecting cognitive development included school readiness, cognitive development, academic achievement, and intelligence (see Appendix for a list of keywords). Publications cited in the reference lists of selected papers and reviews were manually searched. Specialist websites: UNICEF Evaluation Database, UNESCO, World Bank, Brookings Institute, Save the Children, Bernard van Leer, National Institute of Early Education Research (NIEER), Consultative Group on Early Childhood Care and Development, Young Lives, Pratham, International Initiative for Impact Evaluation (3ie), Open Society Institute, and Plan International were searched for conference proceedings, research reports, and policy papers. Since there was a significant overlapping in the nine databases, we decided to search for four key databases in the search of papers published from 2013 to 2016: Academic Search Elite/EBSCOhost, Google Scholar, PsyINFO, and PubMed. A validation search for papers published in 2012 have found that the two searching strategies yielded identical results. After searching the specific keywords in the databases, the titles were firstly screened, which was then followed by abstract screening, and subsequently full-text screening. Six coders worked on these procedures with more than 20% of the screening results randomly cross-checked to ensure inter-rater reliability.
A complete list of inclusion and exclusion criteria can be found in the Appendix. Briefly, studies were included if they were interventions conducted in developing countries (listed by the World Bank as low or middle income countries) and included quantitative evaluations of children’s cognitive development, including global cognitive/mental development (e.g. Mental Development Index of Bayley Scales of Infant Development II), intelligent quotients (e.g. Wechsler Intelligence Scale for Children), language development (e.g. Language Scale in Bayley Scales of Infant and Toddler Development III), executive function (e.g. Stroop Test), academic performance (e.g. standardised language or mathematics test in school), and time to developmental milestone acquisition (e.g. age at which the child can say simple words). Studies targeting special populations, such as Down’s syndrome, cerebral palsy, autism, and any disability, or studies with deficiencies in sampling, data collection, or data analysis after quality assessment were excluded (See Appendix for quality assessment criteria). The current meta-analysis only included those early childhood interventions (either randomised controlled trials, clustered randomised trials, or quasi-experiments) with pure nutritional supplementation, i.e. the difference between intervention and control arms was only nutrient or food supplementation. Interventions were excluded if the effect of supplementation was confounded with other non-supplementation components (e.g. direct stimulation or parenting education).
Data extraction
Intervention was regarded as the unit of analysis. Effect sizes were calculated by comparing differences in cognitive outcomes between intervention and control groups. Multiple relevant outcomes (e.g. Stroop Test and Go/No-Go Test) at a single time point were averaged. Assessment at multiple time points (except for interim analyses before the completion of intervention, which were excluded) were included separately. Unadjusted effect sizes were extracted from randomised controlled trials and adjusted effect sizes were preferably extracted from other studies. The following intervention characteristics were considered as potential effect moderators: type and quantity of nutrients, children’s age (gestational age for antenatal supplementation) at the start of supplementation, and duration of supplementation. Age of cognitive outcome evaluation was also extracted in this study to test whether the effect of nutritional supplementation may change over time. Details on each of the interventions were coded by six coders. Over half of the codes were then randomly selected for verification. At the end of this process, the coding was independently reviewed by all team members.
Statistical analysis
We used the standardised mean difference (Cohen’s d) with Hedges and Olkin’s bias correction as the effect size27. Because of the heterogeneity in cognitive outcome measurements, random-effects meta-analysis models were used with the inverse of effect size precision (variance) as the weighting. Random-effects meta-regression models with restricted maximum likelihood (REML) estimators were used to test the statistical significance of potential moderators28. One meta-regression was constructed for each moderator variable. To address for intra-class correlation due to multiple follow-up time points and multiple publications of a single study, both the study identification number and the intervention identification number were included as random intercepts. Childhood and antenatal interventions were analysed separately. Potential moderators except nutritional content were pre-specified by the authors based on clinical experience and the extant literature. Nutritional content variables were extracted from each intervention and grouped accordingly. Heterogeneity of intervention effect sizes was examined using Cochran’s Q tests and I2 statistics. Publication bias was separately assessed for childhood and antenatal supplementation using funnel plots and Egger regression tests.
References
Bailey, R. L., West, K. P. Jr & Black, R. E. The epidemiology of global micronutrient deficiencies. Ann. Nutr. Metab. 66, 22–33 (2015).
WHO. Guideline: Daily iron and folic acid supplementation in pregnant women (Department of Nutrition for Health and Development, World Health Organization, Geneva, 2012).
Black, R. E. et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 371, 243–260 (2008).
Rao, N., Sun, J., Chen, E. & Ip, P. Effectiveness of interventions to promote early childhood development in developing countries: A systematic review and meta-analysis. Hong Kong J. Paediatr. 22, 14–25 (2017).
Rice, A. L., Sacco, L., Hyder, A. & Black, R. E. Malnutrition as an underlying cause of childhood deaths associated with infectious diseases in developing countries. Bull. World Health Organ. 78, 1207–1221 (2000).
United Nations Children’s Fund. Improving child nutrition: The achievable imperative for global progress. (Division of Communication, UNICEF, 2013).
Grantham-McGregor, S. et al. Developmental potential in the first 5 years for children in developing countries. Lancet 369, 60–70 (2007).
Heckman, J. J. Skill formation and the economics of investing in disadvantaged children. Science 312, 1900–1902 (2006).
Campbell, F. et al. Early childhood investments substantially boost adult health. Science 343, 1478–1485 (2014).
Szajewska, H., Ruszczynski, M. & Chmielewska, A. Effects of iron supplementation in nonanemic pregnant women, infants, and young children on the mental performance and psychomotor development of children: a systematic review of randomized controlled trials. Am. J. Clin. Nutr. 91, 1684–1690 (2010).
Qawasmi, A., Landeros-Weisenberger, A., Leckman, J. F. & Bloch, M. H. Meta-analysis of long-chain polyunsaturated fatty acid supplementation of formula and infant cognition. Pediatrics 129, 1141–1149 (2012).
Eilander, A. et al. Multiple micronutrient supplementation for improving cognitive performance in children: systematic review of randomized controlled trials. Am. J. Clin. Nutr. 91, 115–130 (2010).
Aboud, F. E. & Yousafzai, A. K. Global health and development in early childhood. Annu. Rev. Psychol. 66, 433–457 (2015).
Grantham‐McGregor, S. M., Fernald, L. C., Kagawa, R. & Walker, S. Effects of integrated child development and nutrition interventions on child development and nutritional status. Ann. N. Y. Acad. Sci. 1308, 11–32 (2014).
Larson, L. M. & Yousafzai, A. K. A meta‐analysis of nutrition interventions on mental development of children under‐two in low‐and middle‐income countries. Matern. Child. Nutr. 13, e12229, doi:10.1111/mcn.12229 (2015).
Kristjansson, E. et al. Food supplementation for improving the physical and psychosocial health of socio‐economically disadvantaged children aged three months to five years. Cochrane Database Syst. Rev. 3 (2015).
Muller, O. & Krawinkel, M. Malnutrition and health in developing countries. Can. Med. Assoc. J. 173, 279–286 (2005).
Grantham-McGregor, S. M., Fernald, L. C. & Sethuraman, K. Effects of health and nutrition on cognitive and behavioural development in children in the first three years of life. Part 1: Low birthweight, breastfeeding, and protein-energy malnutrition. Food Nutr. Bull. 20, 53–75 (1999).
McCann, J. C. & Ames, B. N. Is there convincing biological or behavioral evidence linking vitamin D deficiency to brain dysfunction? FASEB J. 22, 982–1001 (2008).
Ramakrishna, T. Vitamins and brain development. Physiol. Res. 48, 175–188 (1999).
Johnson, M. H. Functional brain development in humans. Nat. Rev. Neurosci. 2, 475–483 (2001).
Nelson, C. A. in Handbook of early childhood intervention (eds J. P. Shonkoff & S. J. Meisels) 204-227 (Cambridge University Press, 2000).
Leung, B. M., Wiens, K. P. & Kaplan, B. J. Does prenatal micronutrient supplementation improve children’s mental development? A systematic review. BMC Pregnancy Childbirth 11, 12 (2011).
Copenhagen Consensus Center. Copenhagen Consensus 2012: Expert Panel Findings, http://www.copenhagenconsensus.com/sites/default/files/outcome_document_updated_1105.pdf (2013) (Date of access:27/6/2017).
Rao, N. et al. Early childhood development and cognitive development in developing countries: A rigorous literature review, https://www.gov.uk/government/publications/early-childhood-and-cognitive-development-in-developing-countries (2014) (Date of access:27/6/2017).
Evidence for Policy and Practice Information (EPPI) and Co-ordinating Centre IoE University of London. EPPI-Centre Methods for Conducting Systematic Reviews (EPPI-Centre, London, 2007, updated 2010).
Hedges, L. & Olkin, I. Statistical methods for meta-analysis. (Academic Press, 1985).
Thompson, S. G. & Sharp, S. J. Explaining heterogeneity in meta‐analysis: a comparison of methods. Stat. Med. 18, 2693–2708 (1999).
Acknowledgements
We would like to thank the late Patrice L Engle for her friendship, counsel and contribution to the study design. We also thank the other members of the review team, Jessie Wong, Brendan Weekes, Sheldon Shaeffer, Mark Bray, and Diana Lee. Dr Patrick Ip and Prof Nirmala Rao are the co-corresponding authors, and Mr Frederick K Ho is the co-first author of this article. This paper is based on a literature review, which was commissioned by the Department for International Development, UK in 2013. This additional analysis did not receive any funding support.
Author information
Authors and Affiliations
Contributions
P.I. and N.R. designed the study, interpreted the data, and critically revised the manuscript. F.K.W.H. collected and analysed the data, and drafted the manuscript. J.S. collected and interpreted the data, and critically revised the manuscript. M.E.Y., C.B.C., W.T., and K.L.H. interpreted the data and critically revised the manuscript. All authors approved the final manuscript as submitted.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ip, P., Ho, F.K.W., Rao, N. et al. Impact of nutritional supplements on cognitive development of children in developing countries: A meta-analysis. Sci Rep 7, 10611 (2017). https://doi.org/10.1038/s41598-017-11023-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-11023-4
This article is cited by
-
Nutrient trajectories during infancy and their associations with childhood neurodevelopment
European Journal of Nutrition (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.